Stem Cell Therapy: A Review of the Latest Clinical Evidence in 2024
Stem Cell Research: Introduction
Stem cell therapy has gained popularity as a promising treatment option for conditions where the current medical treatment protocols have been exhausted. However, there is also a lot of confusion due to the overwhelming mixing of credible scientific information and marketing hypes available on the internet.
Due to the fast-changing pace of research and technology, new evidence accumulates rapidly and clinical guidelines need to be periodically updated.
Due to the fast-changing pace of research and technology, new evidence accumulates rapidly and clinical guidelines need to be periodically updated.
As of May 2024, there are more than 300,000 scientific publications published on "stem cell' on the National Library of Medicine (PubMed). More than 9,000 studies have been launched to investigate the potential of stem cell therapy under the U.S. Clinical Trial Registry. There's a lot to read and catch up to. How do we keep up with all these stem cell therapy evidence and research?
www.onedaymd.com
When interpreting scientific studies, let’s remember that not all studies are created equal. Below is a list of study types ranked in descending order based on their evidence quality:
- Meta-analysis and Randomised controlled trials (RCTs)
- Large clinical trials (phase 3)
- Small clinical trials (phase 2) and Case studies
- Mouse results and animal studies
- In Vitro, cell culture, commentary, review, expert opinions and anecdotal evidence
It's important to note that this ranking is not definitive and can vary depending on the specific research question being investigated. Additionally, the quality of individual studies within each type can also vary. Therefore, it's crucial to critically evaluate the methodology and results of any study before drawing conclusions or making decisions based on its findings.
This article contains information and links to stem cell therapy and research in various categories. This list is a work-in-progress list as new evidence might be added from time to time.
Table of Contents:
- Leukemia
- Lymphoma
- Stroke
- Osteoarthritis
- Diabetes
- Spinal Cord Injury
- Autoimmune Diseases
- Heart Diseases
- Autism
- Anti-Aging
- COVID-19
- Kidney Diseases
- Liver Diseases
- Stem Cell and Hair
- Back Pain
If you are new to stem cells, check out stem cell basics.
STEM CELL THERAPY EVIDENCE AND RESEARCH BY CATEGORY
Here, we have listed and compiled all significant scientific publications related to stem cell therapy. The list was compiled by running various searches on PubMed and ClinicalTrials. Each study is hyperlinked to the abstract in the U.S. National Library of Medicine or the full text article to make it easier for healthcare providers or scientists to access more details. The list was complied by running various searches on PubMed.
In order to make it consumer friendly, we have tried to summarise the studies and minimise the technical jargons.
Here is the list by category.
In order to make it consumer friendly, we have tried to summarise the studies and minimise the technical jargons.
Here is the list by category.
1. Leukemia
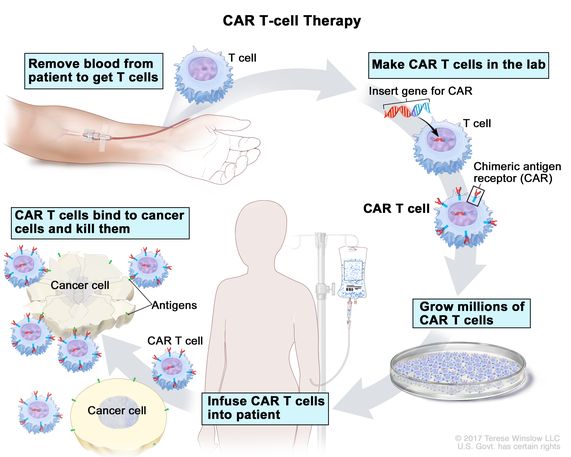
Leukemia is another form of cancer that affects the lymphatic system as well as bone marrow. Leukemia cells are cancerous, affecting the immune system and causing an array of unpleasant symptoms, then eventually leading to death without treatment. It is the most common childhood cancer, but it affects adults of a range of ages as well.There are more than 30,000 publications related to the use of stem cells in leukemia. There are more than 1,000 studies that have been launched to investigate the benefits of stem cell therapy and leukemia. You can review the status and details of these trials on clinicaltrials.gov.
Stem cell therapy poses significant hope, however. Much like with non-Hodgkin’s lymphoma, the treatment involves first killing off leukemia cells with high doses of chemotherapy and sometimes radiation as well. After the majority of cancer cells are defeated, the patient receives an infusion of stem cells to help the body repopulate, so that it can make normal blood cells once again.
This treatment, like the one for non-Hodgkin’s lymphoma, is typically only available to patients who have relapsed. That means their cancer went into remission from standard treatments, then returned months or years later. Good news, though: with a stem cell treatment within the first remission, the survival rate at 5 years is 30-50 percent. If the patient has not experienced a relapse within 2 years of the stem cell transplant, they have a good chance of surviving for many years.
2. Non-Hodgkin’s Lymphoma
Non-Hodgkin’s lymphoma is a type of cancer that arises in the lymphatic system, which is responsible for fighting disease and toxins in the body. White blood cells, also known as lymphocytes, give rise to cancer cells, which then pervade the body. Without treatment, the condition is almost universally fatal.
Chemotherapy is an extremely effective tool against this disease. The problem is, high doses of chemotherapy often kill off bone marrow, in which new blood cells get made. In so doing, the chemotherapy kills cancer but also kills the patient, who now has no source of blood cells.
Stem cell treatment, however, helps mitigate this risk substantially by giving the patient an infusion of new cells following those necessarily high doses of chemotherapy. The patient first receives the chemo, then the stem cell infusion to help them repopulate their blood cell counts. Many patients show great promise of living long and healthy lives following the treatment.
Chemotherapy is an extremely effective tool against this disease. The problem is, high doses of chemotherapy often kill off bone marrow, in which new blood cells get made. In so doing, the chemotherapy kills cancer but also kills the patient, who now has no source of blood cells.
Stem cell treatment, however, helps mitigate this risk substantially by giving the patient an infusion of new cells following those necessarily high doses of chemotherapy. The patient first receives the chemo, then the stem cell infusion to help them repopulate their blood cell counts. Many patients show great promise of living long and healthy lives following the treatment.
There are more than 14,000 publications related to the use of stem cells in lymphoma. There are more than 900 studies that have been launched to investigate the benefits of stem cell therapy and lymphoma. You can review the status and details of these trials on clinicaltrials.gov.
3. Stem Cell Therapy for Stroke
There are more than 3,000 publications related to the use of stem cells and stroke. There are currently more than 60 studies that have been launched to investigate the benefits of stem cell therapy and stroke. You can review the status and details of these trials on clinicaltrials.gov.Stroke constitutes the second leading cause of death worldwide. Stem cell therapy that has been developed over the past several decades represents a promising alternative or supplemental strategy; notably, this approach has already reached the translational stage, with therapeutic results in humans having been discussed in various publications.
In this review (published in Nature), the authors examined the clinical research trends related to stem cell therapy products in the stroke space based on information obtained from the ClinicalTrials.gov website and International Clinical Trials Research Platform (ICTRP) portal site.
Within hours of the stem cell treatment, she was able to move her arms and legs – whereas before she had suffered almost complete immobilization. She and her doctors also noticed rapid improvements in her speech – again, within only a few hours. Other patients noticed astonishing improvements as well, the only side effects coming in the form of “passing headaches.”
4. Stem Cell Therapy for Osteoarthritis
Osteoarthritis is a degenerative condition affecting the joints. Over time, the cartilage that protects joints, preventing the rubbing of one bone on another, breaks down. Eventually, this can lead to the deterioration of the underlying bone as well, causing aching, stiffness, and eventual immobility in many cases. The condition commonly affects the hips, knees, and thumbs, though it can also strike elbows, wrists, ankles, and fingers.
Some doctors and media channels argue that there is very little evidence to support the use of stem cells to treat orthopaedic conditions. However, there are more than 1,700 publications related to the use of stem cells in treating orthopaedic conditions. There are currently more than 120 studies on stem cell treatment for osteoarthritis under the U.S. Clinical Trial Registry.
Several other comparative studies have demonstrated good evidence in the treatment of osteoarthritis. However, there are several approaches and cell lines used. More well-designed and randomised controlled trials are needed to evaluate the best approach and universal consensus. As studies continue, the methods, forms and combinations of stem cell preparations are improving, and outcomes are expected to improve as well.
Stem cell procedure is offered in many clinics within and outside the United States and typically uses adipose cells as the stem cell source. Physicians extract these cells from fat tissue, separate out the stem cells from the rest, then prepare a solution containing growth factors and other ingredients necessary to tell stem cells how to develop in the new site. Once it’s prepared, doctors inject it into the affected site, such as a knee joint.
Regenexx is a U.S. company specializing in orthopedic applications of stem cells that was founded by Dr. Chris Centeno. Dr. Centeno is an expert in the clinical use of mesenchymal stem cells (MSCs) within orthopedic applications. His Regenexx clinic in Denver, Colorado, draws patients from all over the U.S. who are seeking innovative, non-surgical treatments for osteoarthritis, as well as a wide range of other orthopedic applications.
As the visionary behind the revolutionary Regenexx® technology, he pioneered a procedure that involves extracting a small bone marrow sample through a needle and a blood draw from a vein in your arm. These samples are then processed in a laboratory and the stem cells it contains are injected into the area needing repair. The goal is to deliver large numbers of stem cells to the injured area.
Stem cell procedure is offered in many clinics within and outside the United States and typically uses adipose cells as the stem cell source. Physicians extract these cells from fat tissue, separate out the stem cells from the rest, then prepare a solution containing growth factors and other ingredients necessary to tell stem cells how to develop in the new site. Once it’s prepared, doctors inject it into the affected site, such as a knee joint.
Regenexx is a U.S. company specializing in orthopedic applications of stem cells that was founded by Dr. Chris Centeno. Dr. Centeno is an expert in the clinical use of mesenchymal stem cells (MSCs) within orthopedic applications. His Regenexx clinic in Denver, Colorado, draws patients from all over the U.S. who are seeking innovative, non-surgical treatments for osteoarthritis, as well as a wide range of other orthopedic applications.
As the visionary behind the revolutionary Regenexx® technology, he pioneered a procedure that involves extracting a small bone marrow sample through a needle and a blood draw from a vein in your arm. These samples are then processed in a laboratory and the stem cells it contains are injected into the area needing repair. The goal is to deliver large numbers of stem cells to the injured area.
Regenexx, the largest provider of stem cell therapy for orthopaedic conditions in the United States alone, have treated 30,000 patients with stem cell therapy for various joint conditions including knee pain.
Related: Cost of stem cell therapy for knees
www.onedaymd.com
There are more than 3,000 publications related to the use of stem cells in diabetes mellitus. There are currently more than 130 studies that have been launched to investigate the benefits of stem cell therapy and diabetes mellitus. You can review the status and details of these trials on clinicaltrials.gov.
Many people are very interested in the possibility of stem cells to treat diabetes. Both Type I and Type II diabetes have devastating effects on the health of millions, and stem cells may help to ameliorate those conditions.
Type I and Type II diabetes affect the body in different ways. Type I diabetes is genetic, and results from the pancreas failing to produce insulin, or producing too little of it. Insulin is what tells the body to remove glucose from the bloodstream and let it into cells, so they can use it for energy. Most likely this is due to an immune system disorder in which the body attacks its own islets, the pancreatic cells responsible for manufacturing insulin. In this case, stem cells may provide the same immune system-modulating effect as they do for other autoimmune diseases.
Type II diabetes is when the body becomes resistant to insulin. The pancreas may still make it, but the patient’s body does not sense it – it is “insulin resistant,” which means the release of insulin in the bloodstream still does not result in cells taking up glucose. It remains in the bloodstream, causing dangerous hyperglycemia just as it does in the case of Type I.
The second condition may also respond to stem cell treatment, which can help moderate pancreatic productive of insulin as well as helping the body respond to it more effectively. Multiple clinical trials assessing the validity of stem cells for both diseases are underway, and many eagerly await their results.
Type I and Type II diabetes affect the body in different ways. Type I diabetes is genetic, and results from the pancreas failing to produce insulin, or producing too little of it. Insulin is what tells the body to remove glucose from the bloodstream and let it into cells, so they can use it for energy. Most likely this is due to an immune system disorder in which the body attacks its own islets, the pancreatic cells responsible for manufacturing insulin. In this case, stem cells may provide the same immune system-modulating effect as they do for other autoimmune diseases.
Type II diabetes is when the body becomes resistant to insulin. The pancreas may still make it, but the patient’s body does not sense it – it is “insulin resistant,” which means the release of insulin in the bloodstream still does not result in cells taking up glucose. It remains in the bloodstream, causing dangerous hyperglycemia just as it does in the case of Type I.
The second condition may also respond to stem cell treatment, which can help moderate pancreatic productive of insulin as well as helping the body respond to it more effectively. Multiple clinical trials assessing the validity of stem cells for both diseases are underway, and many eagerly await their results.
Currently, two approaches are being used in research, using stem cells as beta-cell producing factories or as a beta cell repair catalyst. Both methods have the same goal which is to return the insulin to normal levels. Diabetes Research Institute (DRI) are running clinical trials and have a number of patients that are living insulin free after receiving a transplant of donor islet cells.
There are many ongoing efforts to understand how stem cell therapy is able to help people with diabetes. One of the main centres is the California Institute of Regenerative Medicine, where you can view the areas of research being conducted specifically to understand diabetes.
A review, published in the Progress in Stem Cell journal in 2019 suggested a combination of antioxidants, growth factors or hormones along with MSCs (mesenchymal stem cells) in optimal combinations and concentrations for the treatment of diabetic nephropathy.
6. Stem Cell Therapy for Spinal Cord Injury
One of the most traumatic injuries to the human body is severing of the spinal cord. Depending on where the injury occurs, the patient may never walk or even move their arms again. For most of human history, such a traumatic injury was completely irreparable. In recent years, neurosurgery has given people back some of their function in cases like these, but outcomes are still all too often disappointing.
Stem cells provide serious hope for the future. Instead of trying to repair damaged nerves, stem cells offer the ability to replace them. By injecting stem cells to the site of the injury, the spinal column can repair itself, accessing all the ingredients it needs for the specialized job.
In combination with growth factors and hormones, stem cells are capable of traveling to the site of the injury “assessing” what needs rebuilding and stepping in to do the job for doctors. This limits the number of modifications needed from the outside and leaves the healing to the body.
While the mechanisms aren’t yet clear, it seems that hormones such as growth factors – in addition to the location in the body – can provide signposts to stem cells telling them what kinds of tissues are needed. Then the stem cells transform into them, integrate with the damaged tissue and repair it.
One of the most traumatic injuries to the human body is severing of the spinal cord. Depending on where the injury occurs, the patient may never walk or even move their arms again. For most of human history, such a traumatic injury was completely irreparable. In recent years, neurosurgery has given people back some of their function in cases like these, but outcomes are still all too often disappointing.
Stem cells provide serious hope for the future. Instead of trying to repair damaged nerves, stem cells offer the ability to replace them. By injecting stem cells to the site of the injury, the spinal column can repair itself, accessing all the ingredients it needs for the specialized job.
In combination with growth factors and hormones, stem cells are capable of traveling to the site of the injury “assessing” what needs rebuilding and stepping in to do the job for doctors. This limits the number of modifications needed from the outside and leaves the healing to the body.
While the mechanisms aren’t yet clear, it seems that hormones such as growth factors – in addition to the location in the body – can provide signposts to stem cells telling them what kinds of tissues are needed. Then the stem cells transform into them, integrate with the damaged tissue and repair it.
There are more than 2,000 scientific publications published related to "stem cell" and "spinal cord injury" on the National Library of Medicine.
There are currently more than 60 studies have been launched to investigate the potential of stem cell therapy for spinal cord injuries under the U.S. Clinical Trial Registry.
There are currently more than 60 studies have been launched to investigate the potential of stem cell therapy for spinal cord injuries under the U.S. Clinical Trial Registry.
Related: Stem Cells and Spinal Cord Injury: Can Stem Cells Help Spinal Cord Injury?
7. Autoimmune Diseases
A huge range of autoimmune conditions exists, such as diabetes, rheumatoid arthritis, multiple sclerosis, lupus, Addison’s disease, Grave’s disease, and more.These conditions all share the characteristic of the body’s immune system reacting to normal substances in the body as though they were pathogenic. That means instead of letting the body function normally, the immune system will attack tissues and substances, creating ongoing sickness and in many cases, eventually death.
Stem cell therapy has two possible benefits in the case of autoimmune diseases. For one thing, it can help repair and regenerative tissues damaged in an autoimmune attack. Stem cells can help them repair nerves, skin, blood, organs, and more. This helps the patient regain their health and fight the degenerative nature of such diseases.
Second, stem cells can actually modulate the immune system so that it no longer attacks the body so viciously – or at all. Research demonstrates that stem cells can minimize the pathological effects of the immune system, making it so the body no longer attacks itself – all while preserving its ability to attack foreign substances and real pathogens.
There are more than 900 scientific publications published related to "stem cell" and "autoimmune disease" on the National Library of Medicine. There are currently more than 200 studies have been launched to investigate the potential of stem cell therapy for autoimmune diseases under the U.S. Clinical Trial Registry.
8. Heart Diseases and Heart Failure
Heart disease is still the No. 1 killer in the United States (although by some estimates cancer will soon or has already surpassed it). For obvious reasons, stem cells seem like a strong possibility for repairing heart tissue and helping to overcome the intermediate symptoms that eventually lead to heart disease or cardiac arrest.As is the case with most of these therapies, the biggest benefit of stem cell treatment for heart disease is its ability to replace damaged or dead cells without the need for invasive surgery or transplants. An injection of stem cells can give the body the ingredients it needs to grow the specialized cells on site, ideally without having to put the patient under or open them up. The exact mechanisms of this procedure are not as yet clear, however.
On 16 May, 2018, Nature News reported that Japan’s health ministry gave doctors at Osaka University permission to take sheets of tissue derived from stem cells and use them to treat diseased human hearts. From preclinical studies in pigs, it appears that thin sheets of cell grafts grown from induced pluripotent stem cells can improve heart function. While the treatment approved by Japan’s health ministry will only be tested in three patients, a follow-up trial could enroll ten or more patients.
There are more than 3,400 scientific publications published related to "stem cell" and "heart failure" on the National Library of Medicine. There are currently more than 200 studies have been launched to investigate the potential of stem cell therapy for spinal cord injuries under the U.S. Clinical Trial Registry.
9. Stem Cell Therapy for Autism
Stem cell therapy for autism is an ongoing topic of research and is considered experimental by the medical community. Parents can find fee-for-service clinics that advertise stem cell therapy for autism, but most of these clinics are operating without FDA approval, and each clinic promotes their own approach, which creates a lot of confusion among parents about how to compare their treatment options.
There are more than 600 publications related to the use of stem cells and autism. There are currently more than 20 studies have been launched to investigate the potential of stem cell therapy for autism under the U.S. Clinical Trial Registry. You can search the database to look for more details of the clinical trials including the countries and centres that are conducting them.
Stem cell therapy for anti aging is an ongoing topic of research and is considered experimental by the medical community. Is there any evidence that stem cell therapy for anti aging is effective and safe?
There are more than 200 publications related to the use of stem cells and anti-aging. There are currently 3 studies that were found for 'stem cell and anti-aging' under the U.S. Clinical Trial Registry.
www.AestheticsAdvisor.com
Despite the fact that there are many published studies on stem cell therapy for anti-aging, major media has been slow to report the findings.
Journal of Gerontology - The results of 2 clinical studies, published in The Journals of Gerontology, showed how a type of adult stem cell called mesenchymal stem cells (MSCs) could reverse the effects of aging.
The first trial involved 15 frail patients, each received single MSC infusion of stem cells collected from adult bone marrow donors aged between 20 and 45 years old. The patients exhibited improved overall quality of life and fitness, as well as diminished tumor necrosis factor levels. The second trial was a double-blind, randomized study involving a placebo group. Aside from noting no adverse effects, the research team found the improvements to be “remarkable.”
www.AestheticsAdvisor.com
Despite the fact that there are many published studies on stem cell therapy for anti-aging, major media has been slow to report the findings.
Journal of Gerontology - The results of 2 clinical studies, published in The Journals of Gerontology, showed how a type of adult stem cell called mesenchymal stem cells (MSCs) could reverse the effects of aging.
The first trial involved 15 frail patients, each received single MSC infusion of stem cells collected from adult bone marrow donors aged between 20 and 45 years old. The patients exhibited improved overall quality of life and fitness, as well as diminished tumor necrosis factor levels. The second trial was a double-blind, randomized study involving a placebo group. Aside from noting no adverse effects, the research team found the improvements to be “remarkable.”
www.AestheticsAdvisor.c
11. Stem Cell Therapy and COVID-19
Can stem cell therapy be used for the treatment of COVID-19? Is it some marketing hype or it's for real.
There are currently more than 1,700 publications related to the use of stem cells and COVID-19. More than 80 studies have been launched to investigate the potential of stem cell therapy for COVID-19 under the U.S. Clinical Trial Registry.
Mesenchymal stem cells are used as immuno-modulators for severe COVID-19 patients and some of the trial projects are launched in combination with other medications such as interleukin (IL) 6 inhibitors e.g. tocilizumab. IL-6 inhibitors have been studied mainly for their potential to calm down the 'cytokine storm' associated with ARDS (acute respiratory distress syndrome), a severe COVID-19 lung complication.
MSCs (mesenchymal stem cells) have recently emerged as a new therapeutic option for hair loss. There are already more than 100 publications related to the use of stem cells and hair loss.
A 2017 study by researchers at Rome University found that 23 weeks after treatment, hair density had improved by a third.
According to Dr Ioannis Liakas, who performs the stem-cell procedure at his London clinic, Vie Aesthetics, it has the potential to not just restore growth but even colour in grey hair.
According to Dr Ioannis Liakas, who performs the stem-cell procedure at his London clinic, Vie Aesthetics, it has the potential to not just restore growth but even colour in grey hair.
There are currently more than 10 studies that were found for 'stem cell and hair loss' under the U.S. Clinical Trial Registry.
13. Stem Cell Therapy for Kidney Diseases
The increasing incidence of kidney diseases raises considerable concerns regarding human health worldwide. A number of studies in recent years have attempted to identify the underlying mechanisms of renal repair in order to explore the potential regenerative capacity of the kidneys. Many papers have reported on the potential use of stem cells of different origins for treating many different pathologies, including kidney diseases.
Several clinical trials have confirmed the safety and tolerability of stem cells, and in particular of MSC-based therapies (Mesenchymal Stem Cell), in patients with renal diseases and kidney transplants (Int J of Mol Sci. 2019).
There are currently more than 100 studies have been launched to investigate the potential of stem cell therapy for kidney disease under the U.S. Clinical Trial Registry. You can search the database to look for more details of the clinical trials including the countries and centres that are conducting them.
14. Stem Cell Therapy for Liver Diseases
There are more than 900 published studies related to the use of stem cells and liver disease and more than 600 published studies related to stem cells and liver failure.
There are currently more than 90 studies were found for 'stem cell and liver disease' under the U.S. Clinical Trial Registry.
A review of 37 studies (Int Journal of Stem Cell Res and Ther. 2017) by Malaysian researchers found that both autologous and allogeneic adult stem cell-based therapies have shown promising results in restoring liver function in cirrhosis patients.
15. Stem Cell Therapy for Back Pain
There are more than 280 published studies related to the use of stem cells and back pain.
Do take note that back pain problems may arise from several causes and degenerative disc disease is just one of the many causes of back pain. Therefore, the importance of finding out the cause first (diagnosis) before treatment.
Stem Cell Clinical Trials for Back Pain and Degenerative Disc Disease
Clinical trials showing the effectiveness of Stem Cell Injections for Lower Back Pain. Most patients in these studies had significant pain relief. Some patients also revealed reversal of disc degeneration. No patients had any serious complications. Since stem cells are a new development in medicine, there is not an abundance of data. However, the data that exists shows that stem cell injections into the disc results in pain relief and improvement in function.
In this landmark study by Pettine’s group (published in 2016), 26 patients with lower back pain had their lumbar discs injected with stem cells which were extracted from the bone marrow. After 2 years, 21 patients (81%) avoided surgery and had pain reduction of 71%. Their function improved by 64%. Additionally, 40% patients had improvements seen on the follow up MRI’s. No complications were reported.
Centeno’s group followed 2372 patients (published in 2016) who had stem cell injections in various joints for 2.2 years. They reported no serious side effects.
Wu’s group performed a metaanalysis (published in 2018) of all the clinical studies regarding stem cells injection into discs and concluded that, Cell-based therapy is for patients who have discogenic low back pain associated with improved pain relief and Oswestry Disability Index.
RELATED:
There are more than 650 published studies related to the use of stem cells and kidney disease and more than 300 published studies related to stem cells and kidney failure.
The increasing incidence of kidney diseases raises considerable concerns regarding human health worldwide. A number of studies in recent years have attempted to identify the underlying mechanisms of renal repair in order to explore the potential regenerative capacity of the kidneys. Many papers have reported on the potential use of stem cells of different origins for treating many different pathologies, including kidney diseases.
Several clinical trials have confirmed the safety and tolerability of stem cells, and in particular of MSC-based therapies (Mesenchymal Stem Cell), in patients with renal diseases and kidney transplants (Int J of Mol Sci. 2019).
There are currently more than 100 studies have been launched to investigate the potential of stem cell therapy for kidney disease under the U.S. Clinical Trial Registry. You can search the database to look for more details of the clinical trials including the countries and centres that are conducting them.
14. Stem Cell Therapy for Liver Diseases
There are more than 900 published studies related to the use of stem cells and liver disease and more than 600 published studies related to stem cells and liver failure.
Liver failure caused by liver cirrhosis, due to various long term liver diseases such as chronic hepatitis B and non-alcoholic fatty liver disease; was thought to be irreversible. A liver transplant is currently the standard treatment to end-stage liver cirrhosis.
However, not every liver failure and liver cirrhosis patients are eligible for a liver transplant in Malaysia. The shortage of matching donors and the high risk of surgical-associated complications further limits the success of a liver transplant.
Since stem cells can be transformed into liver-like cells, the potential of stem cell therapy to treat liver failure and liver cirrhosis has been studied as an interesting new feasible option.
However, not every liver failure and liver cirrhosis patients are eligible for a liver transplant in Malaysia. The shortage of matching donors and the high risk of surgical-associated complications further limits the success of a liver transplant.
There are currently more than 90 studies were found for 'stem cell and liver disease' under the U.S. Clinical Trial Registry.
A review of 37 studies (Int Journal of Stem Cell Res and Ther. 2017) by Malaysian researchers found that both autologous and allogeneic adult stem cell-based therapies have shown promising results in restoring liver function in cirrhosis patients.
15. Stem Cell Therapy for Back Pain
There are more than 280 published studies related to the use of stem cells and back pain.
Do take note that back pain problems may arise from several causes and degenerative disc disease is just one of the many causes of back pain. Therefore, the importance of finding out the cause first (diagnosis) before treatment.
Stem Cell Clinical Trials for Back Pain and Degenerative Disc Disease
Clinical trials showing the effectiveness of Stem Cell Injections for Lower Back Pain. Most patients in these studies had significant pain relief. Some patients also revealed reversal of disc degeneration. No patients had any serious complications. Since stem cells are a new development in medicine, there is not an abundance of data. However, the data that exists shows that stem cell injections into the disc results in pain relief and improvement in function.
In this landmark study by Pettine’s group (published in 2016), 26 patients with lower back pain had their lumbar discs injected with stem cells which were extracted from the bone marrow. After 2 years, 21 patients (81%) avoided surgery and had pain reduction of 71%. Their function improved by 64%. Additionally, 40% patients had improvements seen on the follow up MRI’s. No complications were reported.
Centeno’s group followed 2372 patients (published in 2016) who had stem cell injections in various joints for 2.2 years. They reported no serious side effects.
Wu’s group performed a metaanalysis (published in 2018) of all the clinical studies regarding stem cells injection into discs and concluded that, Cell-based therapy is for patients who have discogenic low back pain associated with improved pain relief and Oswestry Disability Index.
There are currently more than 10 studies that have been launched to investigate the potential of 'stem cell therapy for back pain' under the U.S. Clinical Trial Registry. You can search the database to look for more details of the clinical trials including the countries and centres that are conducting them.
There are 15 spinal cord pathology studies listed on www.clinicaltrials.gov with only two studies completed, four recruiting, four status-unknown, one suspended, two active-not-recruiting, one withdrawn and one terminated. Although some preliminary information has been obtained, much remains to be determined with respect to the best method of stem cell delivery, source of stem cell, numbers of cells to be delivered and the patient selection to receive such therapy. These considerations are common to all potential spine-related stem cell applications.
There are four FDA approved adipose derived stem cell clinical trials at Sanford Health: osteoarthritis of the knee, osteoarthritis of the wrist, rotator cuff tear, and facet joints.
There is a product currently in phase 3 clinical trials at the US Food and Drug Administration (FDA) called Mesoblast. If the results from that study are favourable, then we could have stem cells available for the treatment of degenerative disc disease very soon.
Another clinical trial that has just completed it's recruitment, studied Mesenchymal Precursor Cells (MPCs) in 100 Subjects With Lumbar Back Pain.
The iPSpine project, a pan-European clinical trial was awarded €15 million in 2019 under the Horizon 2020 programme, towards researching a solution for chronic lower back pain. The huge public-private consortium is comprised of 20 partners, and is coordinated by the Faculty of Veterinary Medicine of the Utrecht University (Netherlands).
The iPSpine consortium was formed to initiate a European-led research effort to identify a future advanced therapeutic strategy that can address the societal need for a radical new treatment of IDD-induced LBP (Intervertebral Disc Degeneration - induced Low Back Pain).
The aim of iPSpine is to investigate and develop a new advanced therapy medicinal product (ATMP) of the future, based on a novel developmental biology approach involving induced pluripotent stem cells (iPSC) and smart biomaterials.
There are 15 spinal cord pathology studies listed on www.clinicaltrials.gov with only two studies completed, four recruiting, four status-unknown, one suspended, two active-not-recruiting, one withdrawn and one terminated. Although some preliminary information has been obtained, much remains to be determined with respect to the best method of stem cell delivery, source of stem cell, numbers of cells to be delivered and the patient selection to receive such therapy. These considerations are common to all potential spine-related stem cell applications.
There are four FDA approved adipose derived stem cell clinical trials at Sanford Health: osteoarthritis of the knee, osteoarthritis of the wrist, rotator cuff tear, and facet joints.
There is a product currently in phase 3 clinical trials at the US Food and Drug Administration (FDA) called Mesoblast. If the results from that study are favourable, then we could have stem cells available for the treatment of degenerative disc disease very soon.
Another clinical trial that has just completed it's recruitment, studied Mesenchymal Precursor Cells (MPCs) in 100 Subjects With Lumbar Back Pain.
The iPSpine project, a pan-European clinical trial was awarded €15 million in 2019 under the Horizon 2020 programme, towards researching a solution for chronic lower back pain. The huge public-private consortium is comprised of 20 partners, and is coordinated by the Faculty of Veterinary Medicine of the Utrecht University (Netherlands).
The iPSpine consortium was formed to initiate a European-led research effort to identify a future advanced therapeutic strategy that can address the societal need for a radical new treatment of IDD-induced LBP (Intervertebral Disc Degeneration - induced Low Back Pain).
The aim of iPSpine is to investigate and develop a new advanced therapy medicinal product (ATMP) of the future, based on a novel developmental biology approach involving induced pluripotent stem cells (iPSC) and smart biomaterials.
Stem Cell Therapy for Other Indications
The public may search a database of NIH-sponsored clinical trials at www.clinicaltrials.gov. Enter the search terms of interest (e.g., Parkinson's Disease and stem cells) to search for applicable clinical trials.
The public may search a database of NIH-sponsored clinical trials at www.clinicaltrials.gov. Enter the search terms of interest (e.g., Parkinson's Disease and stem cells) to search for applicable clinical trials.
Find leading stem cell centers within the U.S. and worldwide.
We covered six leading stem cell centers: Best Stem Cell Clinics.Each one has treated large populations of patients with adult stem cells. Several of them, have been treating patients for over a decade.
Please note that we are not advising patients to seek treatment from these companies. We are identifying them to allow readers to seek out more information and discuss with their trusted healthcare providers.
Please note that we are not advising patients to seek treatment from these companies. We are identifying them to allow readers to seek out more information and discuss with their trusted healthcare providers.
Conclusion
If there are any new major stem cell related evidence that we’ve missed, please let us know in the comments and we’ll add them to the post! Thank you in advance.
If there are any new major stem cell related evidence that we’ve missed, please let us know in the comments and we’ll add them to the post! Thank you in advance.



Comments
Post a Comment